Lithium metal is one of the best candidates to replace graphite as an anode material thanks to its high theoretical capacity. The problem is that batteries using lithium metal anodes currently have poor cycle life.
However, thanks to a new non-flammable dual-anion ionic liquid electrolyte this could soon change.
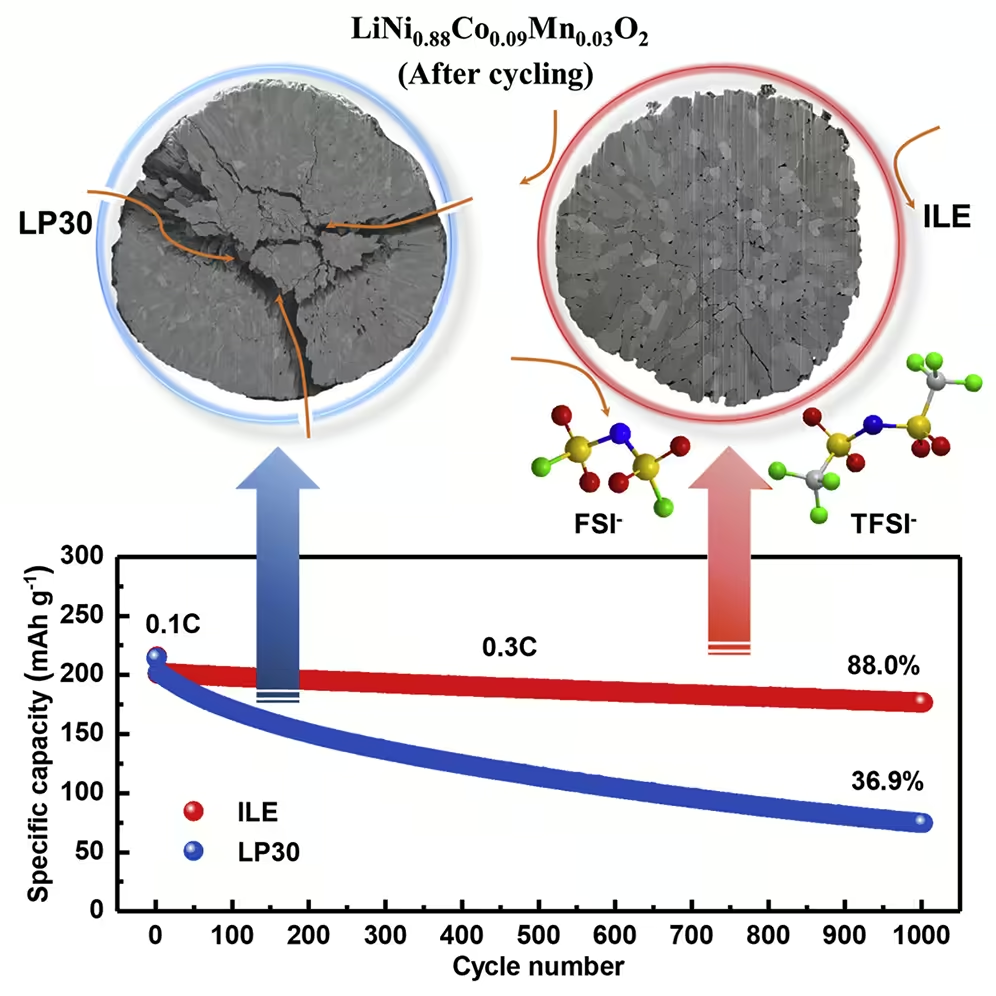
Researchers at the Karlsruhe Institute of Technology (KIT) and the Helmholtz Institute Ulm – Electrochemical Energy Storage (HIU) have now found a solution. As you report in Joule magazine, you are using a promising new combination of materials. They use a low-cobalt, nickel-rich layered cathode (NCM88). This offers a high energy density. With the commonly used commercially available organic electrolyte (LP30), however, the stability leaves a lot to be desired. The storage capacity decreases as the number of charging cycles increases.
Why this is so, explains Professor Stefano Passerini, Director of the HIU and head of the battery electrochemistry research group: “In the LP30 electrolyte, particle cracks occur on the cathode. The electrolyte reacts within these cracks and destroys the structure. In addition, a thick, moss-like lithium-containing layer forms on the cathode. ”The researchers therefore used a non-volatile, non-flammable ionic liquid electrolyte with two anions (ILE) instead. “With the help of the ILE, the structural changes in the nickel-rich cathode can be significantly reduced,” reports Dr. Guk-Tae Kim from the Battery Electrochemistry Research Group at HIU.
88 percent capacity retained over 1,000 charge cycles
The results: With the cathode NCM88 and the electrolyte ILE, the lithium metal battery achieves an energy density of 560 watt hours per kilogram (Wh / kg). It initially has a storage capacity of 214 mAh per gram (mAh / g); 88 percent of the capacity is retained over 1,000 charging cycles. The Coulomb efficiency, which indicates the ratio between withdrawn and supplied capacity, averages 99.94 percent.
Since the presented battery is also characterized by a high level of safety, the researchers from Karlsruhe and Ulm have thus taken an important step on the way to carbon-neutral mobility.
Initially I didn’t notice that the energy density figure of 560 Wh/kg advertised on the press release only considered the weight of the active material (cathode and anode), so thanks Andrés for the heads up!
The research paper clarifies it.
The resulting specific energy based on the overall (anode + cathode) active material weights is calculated to be 564 Wh kg−1 at 0.1C, and 488 Wh kg−1 at 0.5C using the thin Li electrode.
Highlights
- Energy density of 560 Wh/kg (only active material considered)
- Capacity retention of 88 % after 1.000 cycles
- NCM 88 (low cobalt content) cathode
- Safe non-flammable electrolyte
More info: